LTR Medical and Medovate, the manufacturer of SAFIRA™ have signed an agreement which will allow us to assess the potential for distribution of SAFIRA™ for veterinary use across Australia and New Zealand.
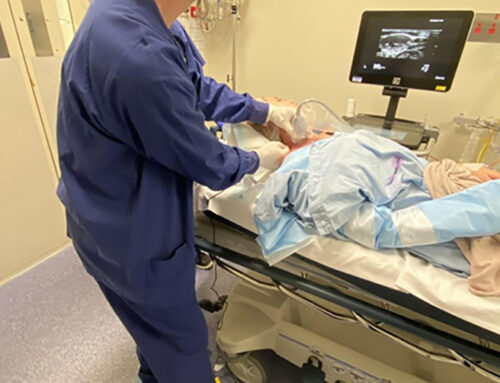
SAFIRA™ – a revolutionary new technology that transforms regional anaesthesia into a one-person procedure – was originally developed for human patient use in hospitals, but there is huge potential for its unique benefits to be extended to veterinary regional anaesthesia.
Over the coming months we will work to engage with veterinary practices to secure evaluations of SAFIRA™ for veterinary applications. The studies will complement the UK studies that are already underway providing further insights and evidence of the potential for SAFIRA™ in small animal anaesthesia.
SAFIRA™ incorporates a unique safety solution that limits injection pressure, helping to reduce the risk of nerve injury. In current regional anaesthesia procedures, two operators are often needed, including a second operator (an assistant) who injects the anaesthetic solution at the required pressure, which relies on ‘feel’. This means anaesthetic solutions can be injected at unsafe pressures.
Another huge benefit for animal care is that the device enables a slow, controlled release of local anaesthetic, enabling the lower amounts of anaesthetic to be used to achieve a successful nerve block.
Danny Zanardo, VP of Commercial for LTR Medical commented: “There are a significant number of regional anaesthesia nerve blocks performed on animals every year and LTR Medical are excited to introduce SAFIRA™ into this market throughout Australia and New Zealand. Use of SAFIRA™ can lead to less anaesthetic being used on animals and improved quality of recovery.”
Regional anaesthesia is an important part of small animal care and is used for many reasons in veterinary medicine, including improved quality of recovery. The use of ultrasound guided regional anaesthesia is becoming more common practice today, with the technique – which can help provide a higher level of visualisation for the practitioner – increasingly seen as a ‘gold standard’.
Chris Rogers, Sales & Marketing Director at Medovate, commented: “This is a very exciting opportunity to assess how SAFIRA’s unique safety benefits can be translated into small animal regional anaesthesia and thus introduced into wider veterinary practice. We look forward to the evaluations which will hopefully add to the growing repository of evidence for the application of SAFIRA™ in veterinary care.”
SAFIRA™ has been successfully launched in the human healthcare field in the USA, Australia and New Zealand, bringing to market Medovate’s first medtech innovation.